Aromatic thioketone-mediated radical polymerization of methacrylates and the preparation of amphiphilic quasi-block copolymers†
Abstract
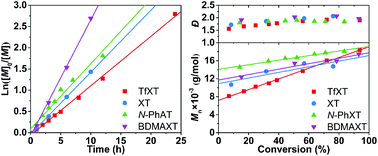
Four aromatic thioketones with substituents exhibiting widely different electronic effects, 2-trifluoromethyl-9H-xanthene-9-thione/TfXT, 9H-xanthene-9-thione, 10-phenylacridine-9(10H)-thione and 2,7-bis(dimethylamino)-9H-xanthene-9-thione, were successfully synthesized. Their effects on the radical polymerization of methyl methacrylate (MMA) were evaluated and compared. All polymerizations displayed pseudo-first-order and decelerated kinetics, with the deceleration effect enhancing when the substituents were changed from a strong electron-donating –N(CH3)2 group to a strong electron-withdrawing –CF3 group. TfXT outweighed the other thioketones and could keep the polymerization under good control when the equivalence of TfXT to the initiator was higher than 2. Next, electrospray ionization mass spectroscopy (ESI-MS) was conducted to investigate the underlying mechanism of the present method, which involves the rapid spin trapping of propagating radicals by TfXT and subsequent cross-termination with other propagating radicals. Finally, the present method was applied in the synthesis of an amphiphilic quasi-block copolymer of MMA and methacrylic acid following a one-pot two-step approach. This method contributes towards designing CLRP with improved controlled/living features and compatibility with industrial processes.


 Please wait while we load your content...
Please wait while we load your content...