Highly active bimetallic nickel catalysts for alternating copolymerization of carbon dioxide with epoxides†
Abstract
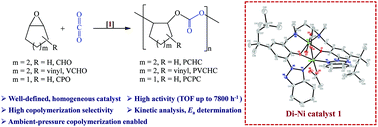
We reported a series of well-characterized di-nuclear nickel catalysts containing 1,3-diamine-bis(benzotriazole phenolate) derivatives for high-performance copolymerization of CO2 and epoxides. The di-nickel di-acetate complex 1 was found to be a highly active catalyst to alternatingly copolymerize CO2 with cyclohexene oxide (CHO), producing poly(cyclohexene carbonate)s (PCHCs) with a high turnover frequency (TOF) up to 7800 h−1 under optimized conditions. Interestingly, complex 1 also appears to be the first example of nickel-catalyzed CO2-copolymerization with CHO that could proceed at 1 atm CO2 pressure. Not only has the excellent catalysis of CO2/CHO copolymerization by di-Ni 1 been enabled, but also 1 has been further applied to effectively catalyze the copolymerization of CO2 with 4-vinyl-1,2-cyclohexene oxide or cyclopentene oxide (CPO) to obtain the corresponding polycarbonates. Particularly, 1 was also reported for the first time to be highly efficient for nickel-catalyzed CO2/CPO copolymerization and enabled the formation of poly(cyclopentene carbonate)s with a TOF > 700 h−1 and excellent carbonate linkage contents (>99%). Kinetic studies of the Ni-catalyzed CO2-copolymerization of CHO were performed and the activation energy for PCHC formation by 1 is 60.6 kJ mol−1.


 Please wait while we load your content...
Please wait while we load your content...