A high performance phenyl-free LED photoinitiator for cationic or hybrid photopolymerization and its application in LED cationic 3D printing†
Abstract
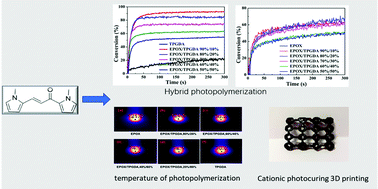
In this work, a high performance LED photoinitiator, 1,3-bis(1-methyl-1H-pyrrol-2-yl)prop-2-en-1-one (BMO), without a benzene ring was synthesized through a one-step aldehyde–ketone condensation reaction. BMO has a broad absorption range and a high molar extinction coefficient from 365 to 425 nm. Interestedly, BMO can be used not only as a type II free radical photoinitiator for which the hydrogen donor can be supplied by itself or other tertiary amines, but also as a long wavelength sensitizer to sensitize diphenyliodonium photoinitiators (Iod) under a 365 nm to 425 nm LED lamp. BMO/Iod can initiate cationic and free radical photopolymerization. It also can initiate the hybrid photopolymerization efficiently. As a sensitizer to Iod, its activity in initiating cationic photopolymerization is not less than that of 2-isopropylthioxanthone (ITX). In particular, it remains highly active at a low light intensity under a LED lamp. This is very significant to the cationic photopolymerization as well as the photocuring 3D printing technique.


 Please wait while we load your content...
Please wait while we load your content...