Organobase-catalysed hydroxyl–yne click polymerization†
Abstract
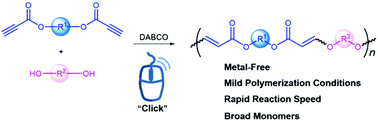
Alkyne-based click polymerization has become a powerful tool for the preparation of functional polymers. However, the click polymerizations performed under mild reaction conditions should be further developed for more facile synthesis of functional polymeric materials. In this work, an efficient hydroxyl–yne click polymerization using ester activated alkynes and alcoholic hydroxyl groups as monomers is developed in the presence of a commercially available organobase bicyclo[2.2.2]-1,4-diazaoctane (DABCO) under ambient conditions. Thermally stable poly(vinyl ether ester)s (PVEEs) with high weight-average molecular weights (up to 71 000) are produced in excellent yields (up to 99%). Moreover, both semi-crystalline and amorphous polymers are obtained depending on the different flexibilities of monomers. By incorporating an aggregation-induced emission (AIE) moiety of tetraphenylethylene (TPE) into the main chains, the corresponding polymers show typical AIE features. This work not only establishes an efficient DABCO-catalysed hydroxyl–yne click polymerization but also enriches the family of click polymerizations.


 Please wait while we load your content...
Please wait while we load your content...