Ambipolar polyimides with pendant groups based on 9H-thioxanthene-9-one derivatives: synthesis, thermostability, electrochemical and electrochromic properties†
Abstract
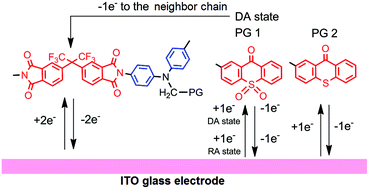
New electro-active polyimides (PI) with pendant groups based on 9H-thioxanthene-9-one (Th(O)S) and its S,S-dioxide derivative (Th(O)SO2) have been synthesized on the basis of 2-{bis(4-aminophenyl)aminomethyl}-9H-Th(O)S/Th(O)SO2 by the route including the synthesis of the corresponding polyamidoacids followed by their chemical imidization. The synthesized polyimides demonstrated thermal stability up to 400 °C without a noticeable weight loss. Thin-layer cyclic voltammetry showed the polyimides to be capable of reversible electron transfer at low negative potentials, their values depending on the nature of the pendant groups. The electrochromic behavior of the polyimides showed absorption bands at 363, 409, 683 nm for the PI with the Th(O)S pendant group and 355, 644 nm for the PI with the Th(O)SO2 in the potential sweep range 0 > E > −2.1 V. Optical absorption bands under electrochemical reduction conditions are associated with the formation of radical anion states of the pendant groups inside the PI layer as well as reversible electron transfer to the electron-acceptor moiety of the polymer chain. It was confirmed by comparison with 3D UV-VIS-NIR spectroelectrochemical studies of the corresponding 2-methyl-9H-thioxanthene-9-ones used as precursors of the pendant groups.


 Please wait while we load your content...
Please wait while we load your content...