Manipulating the helix–coil transition profile of synthetic polypeptides by leveraging side-chain molecular interactions†
Abstract
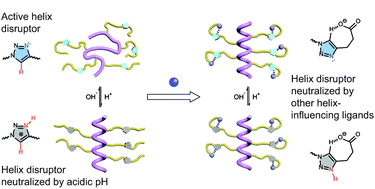
Polypeptides with trigger-responsive helix–coil transition behaviours are interesting biomaterials due to their helix-specific assemblies and biomedical performances. Based on the pH-sensitive, conformationally switchable triazole polypeptides, we reported the manipulation of the helix–coil transition profile, which was determined by the combined molecular interactions of triazole and other side-chain functionalities. Specifically, the introduction of side-chain hydrophobic moieties or hydrogen bonding acceptors neutralized the helix-disrupting effect of side-chain triazoles, which altered the pH-responsive conformational transition profile of the polypeptides. These results inspired us to design new triazole polypeptides bearing dimethylamino side chains, which exhibited interesting helix–coil–helix transition behaviours as the pH decreased.


 Please wait while we load your content...
Please wait while we load your content...