Anionic polymerization of acrylic thioester by using organic base†
Abstract
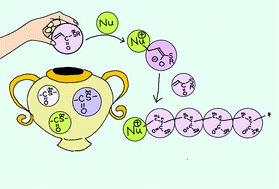
The unique features of thioesters prompted us to study the anionic polymerization of acrylic thioesters by using organic bases. S-(4-t-Butylbenzyl) thioacrylate was polymerized with DBU (1,8-diazabicyclo[5.4.0]undec-7-ene), MTBD (7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene), TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene), DMAP (4-dimethylaminopyridine), and DABCO (1,4-diazabicyclo[2.2.2]octane) as initiators for zwitterion formation. Propagation occurred at the anionic sites of the zwitterions, and an aprotic polar solvent, namely DMF, favored the polymerization. Polymers with Mn = 3700–55 800 and Mw = 11 900–156 800 were obtained in the presence of 2–10 mol% of the bases at room temperature to −40 °C in DMF. Higher-molecular-weight polymers were produced at lower temperatures and by using a smaller amount of the base. However, because chain-transfer reactions were involved, the PDI was 2.3–3.5; this was also the result of slow initiation with fast propagation. Addition of a Li salt gave lower-molecular-weight polymers with smaller PDI values (<2.0). This is presumably the result of stabilization of the propagating anion by the Li cation. However, the polymer terminal structures identified by ESI-MS suggested that chain-transfer reactions were still involved.


 Please wait while we load your content...
Please wait while we load your content...