Synthesis of a soluble adenine-functionalized polythiophene through direct arylation polymerization and its fluorescence responsive behavior†
Abstract
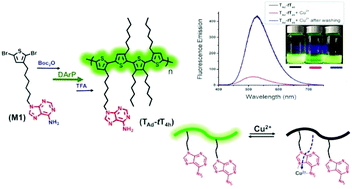
Side chain engineering has been used widely to expand the functionality and enhance the solubility of conjugated polymers, promoting their utility in various applications. Herein, we report the synthesis of an adenine-functionalized, thiophene-based alternating copolymer via direct arylation polymerization. This nucleobase-modified, alkyl thiophene-based alternating copolymer was accessed by copolymerization of a Boc-protected, adenine functionalized thiophene monomer 9-(6-(2,5-dibromothiophen-3-yl)hexyl)-9H-purine-6-amine (TAd), with 3,3′,3′′,4′-tetrahexyl-2,2′:5′,2′′-terthiophene, (tT4h). Quantitative post-polymerization deprotection of Boc groups results in the adenine bearing alternating copolymer (TAd-tT4h), which is soluble in common organic solvents due to steric hindrance-induced flexibility of the tT4h comonomer, allowing structure–property relationships to be established. In comparison to the unfunctionalized analogue, interchain hydrogen bonding through the adenine functionality enhances the packing of the copolymer, resulting in a ∼70 °C increase in the glass transition temperature. Furthermore, the improved solubility of the copolymer and capacity for strong metal ion binding by the nucleobase leads to dramatic fluorescence quenching (>90%) upon addition of Cu2+ ions, which is also reflected in a high Stern–Volmer constant of 1.28 × 104 M−1. The fluorescence emission is recovered almost completely after washing the copolymer solution with EDTA-disodium salt aqueous solution. These findings demonstrate the viability of synthesizing soluble, fully conjugated copolymers with nucleobase functionality via direct arylation polymerization, as well as the influence of the hydrogen bonding nucleobase on thermal, optical, and metal-ion sensing properties.


 Please wait while we load your content...
Please wait while we load your content...