Synthesis of modifiable photo-responsive polypeptides bearing allyloxyazobenzene side-chains†
Abstract
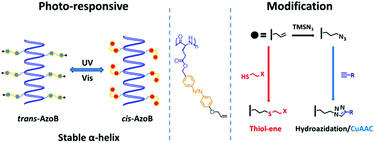
Synthetic polypeptides are important protein mimics due to their similar primary and secondary structures. Previously, the properties of polypeptides can be regulated by either introducing N-carboxyanhydride (NCA) stimuli-responsive moieties directly or modifiable groups for post-polymerization functionalization. Here we combine the two approaches and report the synthesis of photo-responsive and modifiable helical poly(L-glutamate) bearing an azobenzene (Azo) and an alkene (“ene”) group in the side chain, namely P(AzoEne-Glu)s. The polypeptides, prepared by the controlled ring-opening polymerization of a novel monomer AzoEne-GluNCA, undergo rapid and high yield UV-triggered trans–cis isomerization of the Azo moiety. However, this change in the primary structure does not lead to interruption of the helical secondary structure. The rigid helical main-chain and pendent Azo mesogen together endow P(AzoEne-Glu)s with liquid crystalline properties. Moreover, the “ene” group of P(AzoEne-Glu)s can be modified with thiols via a UV-mediated thiol–ene reaction, or quantitatively transformed to azide via a novel anti-Markovnikov hydroazidation approach. The newly generated azido-containing polymer can be further converted by click-type cycloaddition reactions. This work may streamline the rapid synthesis of diverse functional and stimuli-responsive polypeptides that are potentially useful for self-assembly and liquid crystalline materials.
- This article is part of the themed collection: Polymer Chemistry Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...