The effect of deuteration on the keto–enol equilibrium and photostability of the sunscreen agent avobenzone†
Abstract
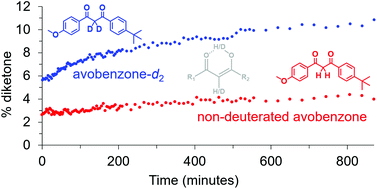
The remarkable properties of deuterium have led to many exciting and favourable results in enhancing material properties, for applications in the physical, medical, and biological sciences. Deuterated isotopologues of avobenzone, a sunscreen active ingredient, were synthesised to examine for any changes to the equilibrium between the diketone and enol isomers, as well as their UV photostability and photoprotective properties. Prior to UV irradiation, deuteration of the diketone methylene/enol moiety (i.e. avobenzone-d2) led to an increase in the % diketone compared to non-deuterated, determined by 1H NMR experiments in CDCl3 and C6D12. This can be rationalised from two angles; mechanistically by a deuterium kinetic isotope effect for the CH vs. CD abstraction step during tautomerisation from the diketone to the enol, and a weaker chelating hydrogen bond for the enol when deuterated allowing increased equilibration to the diketone. Avobenzone-d2 was further examined by solid state 13C NMR. The higher % diketone for avobenzone-d2 was postulated to favour increased photodegradation by a non-reversible pathway. This was investigated by UV irradiation of the avobenzone isotopologues in C6D12, both in real time in situ within the NMR by fibre optic cable as well as ex situ using sunlight. An increase in the relative amount of photoproducts for avobenzone-d2 compared to non-deuterated was observed by 1H NMR upon UV irradiation ex situ. Overall, the study demonstrates that deuteration can be applied to alter complex equilibria, and has potential to be manifested as changes to the properties and behaviour of materials.


 Please wait while we load your content...
Please wait while we load your content...