Advances in Cu and Ni-catalyzed Chan–Lam-type coupling: synthesis of diarylchalcogenides, Ar2–X (X = S, Se, Te)
Abstract
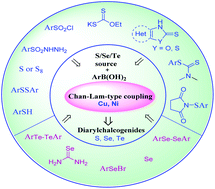
Organochalcogenides are an important class of compounds encountered in organic synthesis, chemical biology and materials chemistry. The development of the Ar2–S/Se/Te bond formation, mostly in the synthesis of diarylchalcogenides, has garnered considerable attention in recent years. Transition metal catalysis is one of the most used methods for the synthesis of diarylchalcogenides via the traditional cross-coupling strategy. Recently, the Chan–Lam coupling has been valued to be the best alternative for the traditional cross-coupling due to the mild and effective reaction conditions. The review summarizes the strategies adopted for the synthesis of diarylchalogenides (Ar2X, X = S, Se, Te) by Chan–Lam-type coupling using Cu or Ni catalysis and comprises diverse approaches based on different sources of arylsulfur donors like thiols, diaryldisulfides, molecular sulfur, diheteroaryldisulfides and similar sources of Se and Te.


 Please wait while we load your content...
Please wait while we load your content...