Regioselectivity in the adiabatic photocleavage of DNA-based oxetanes†
Abstract
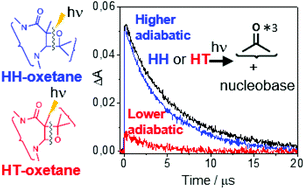
Direct absorption of UVB light by DNA may induce formation of cyclobutane pyrimidine dimers and pyrimidine–pyrimidone (6-4) photoproducts. The latter arise from the rearrangement of unstable oxetane intermediates, which have also been proposed to be the electron acceptor species in the photoenzymatic repair of this type of DNA damage. In the present work, direct photolysis of oxetanes composed of substituted uracil (Ura) or thymine (Thy) derivatives and benzophenone (BP) have been investigated by means of transient absorption spectroscopy from the femtosecond to the microsecond time-scales. The results showed that photoinduced oxetane cleavage takes place through an adiabatic process leading to the triplet excited BP and the ground state nucleobase. This process was markedly affected by the oxetane regiochemistry (head-to-head, HH, vs. head-to-tail, HT) and by the nucleobase substitution; it was nearly quantitative for all investigated HH-oxetanes while it became strongly influenced by the substitution at positions 1 and 5 for the HT-isomers. The obtained results clearly confirm the generality of the adiabatic photoinduced cleavage of BP/Ura or Thy oxetanes, as well as its dependence on the regiochemistry, supporting the involvement of triplet exciplexes. As a matter of fact, when formation of this species was favored by keeping together the Thy and BP units after splitting by means of a linear linker, a transient absorption at ∼400 nm, ascribed to the exciplex, was detected.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...