Iodine assisted synthesis of CF3 appended spirodihydrofuryl/cyclopropyl oxindoles by changing the active methylene sources†
Abstract
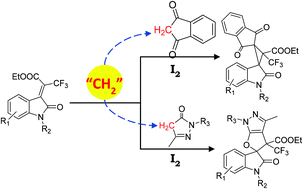
This paper describes the synthesis of two distinct types of CF3-containing spirooxindoles by varying the active methylene sources. The reaction was carried out in DMSO, assisted by molecular iodine and Na2CO3via systematic application of Michael reaction and iodine mediated cyclisation. With 5-methyl-2,4-dihydro-3H-pyrazol-3-one as the methylene source, the final products obtained were spirodihydrofuropyrazolyl oxindoles, whereas 1H-indene-1,3(2H)-dione as the methylene source gave the final compounds spirocyclopropyl oxindoles. Modest to good yields were obtained for both the spiro systems.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...