Abstract
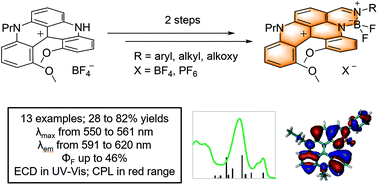
A series of chiral fluorescent helicene-BODIPY conjugates was prepared by the regioselective formylation of aza[4]helicene precursors and then an efficient one-pot two-step BODIPY synthesis (13 examples, 28–82%). Fused conjugates exhibit absorption and fluorescence properties (ΦF 30–45%) in the red visible domain, and a CPL signature could be measured at 605 nm (glum ±5 × 10−4). Photophysical and electronic properties were investigated and rationalized through first principles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...