β-Borylation of conjugated carbonyl compounds with silylborane or bis(pinacolato)diboron catalyzed by Au nanoparticles†
Abstract
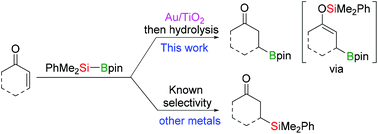
Conjugated aldehydes and ketones undergo reaction with Me2PhSiBpin (pin: pinacolato) catalyzed by Au nanoparticles supported on TiO2 forming exclusively the β-borylation products, via the intermediate formation of the labile silaboration adducts. This chemoselectivity pathway is complementary to the so far known analogous reaction catalyzed by other metals, where β-silylation occurs instead. β-Borylation also occurs with pinBBpin under identical reaction conditions in a variety of conjugated carbonyl compounds, including esters and amides which are unreactive in their attempted Au-catalyzed silaboration.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...