Catalyst-free 1,6-conjugate addition of indoles and 4-hydroxycoumarins to para-quinone methides: synthesis of unsymmetrical triarylmethanes†
Abstract
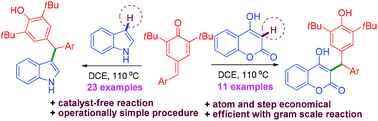
The catalyst-free 1,6-conjugate addition of indoles and 4-hydroxycoumarins to para-quinone methides is reported. This protocol allowed us to access a range of unsymmetrical triarylmethanes in good to excellent yields. The outlined procedure is operationally simple, efficient, atom and step economical. The synthesized heterocyclic triarylmethanes were further converted into highly substituted indoloisoquinolines and pyranochromenones via metal-catalyzed C–H activation/annulation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...