The base-induced regioselective radical arylation of 3-aminochromone with aryl hydrazine†
Abstract
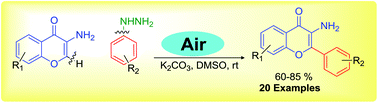
A simple and efficient direct radical C-2 arylation of 3-aminochromone derivatives with aryl hydrazine is described. The aryl hydrazine acts as an initiator and source for the aryl radical via the cleavage of the C–N bond of aryl hydrazine. The reaction proceeds via a base-promoted single electron transfer (SET) pathway. The aryl radical abstracts a single electron from 3-aminochromone, which generates a C2-radical iminium ion to undergo a cross-coupling reaction with an aryl radical, and this process offers an array of regioselective 2-aryl substituted 3-aminochromones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...