Experimental and computational evidence on gold-catalyzed regioselective hydration of phthalimido-protected propargylamines: an entry to β-amino ketones†
Abstract
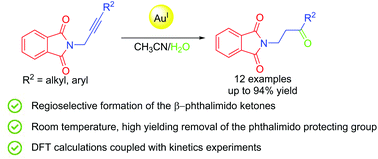
The results of our investigations on the Au-catalyzed regioselective hydration reaction of both alkyl- and aryl-substituted N-propargyl phthalimides directed to the selective formation of the corresponding β-phthalimido ketones are described. Experimental data, in particular the observed regioselectivity, have been qualitatively supported by quantum-chemical calculations carried out on model systems in the framework of Density Functional Theory (DFT) followed by quantum theory of atoms in molecules (QTAIMS). Our results suggest that the electronic features of the initial adduct between the propargyl triple bond and the Au(I) catalyst, in particular the character of the gold-triple bond interaction, are essential for the observed regioselectivity. Other effects, such as the presence of the solvent and the formation of a H-bond between the water molecule and the phthalimido moiety, although apparently irrelevant for the regioselectivity, have proven to be kinetically and catalytically rather important.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...