An efficient imidation of thioethers with nitrene in water†
Abstract
The first imidation of thioethers with free nitrene in water was realized. N-Cbz sulfilimines are formed via imidation of thioethers with free nitrene generated from α elimination of nosyloxycarbamates. In this work, water is successfully applied as solvent for free nitrene, and transition metal catalyst is not needed.
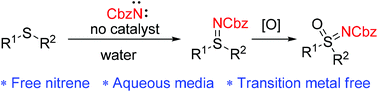
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...