Synthesis of 1-benzylisoindoline and 1-benzyl-tetrahydroisoquinoline through nucleophilic addition of organozinc reagents to N,O-acetals†
Abstract
A new approach to access 1-benzylisoindoline and 1-benzyl-tetrahydroisoquinoline has been developed through nucleophilic addition of organozinc reagents to N,O-acetals. A number of substituted organozinc reagents were amenable for this transformation, and the desired products were obtained with excellent yields. Moreover, Sc(OTf)3 proved to be an effective catalyst for the formation of 1-benzylisoindoline and 1-benzyl-tetrahydroisoquinoline using such nucleophilic addition.
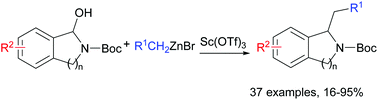
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...