Anion–cation synergistic metal-free catalytic oxidative homocoupling of benzylamines by triazolium iodide salts†
Abstract
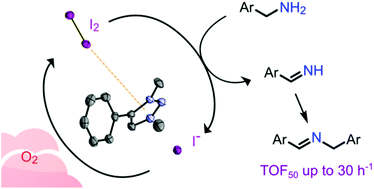
Triazolium iodide salts are excellent catalysts for the selective oxidative coupling of benzylamines to yield imines. This metal-free reaction proceeds in quantitative spectroscopic yields when run in refluxing 1,2-dichlorobenzene and open to the air. No catalytic activity was observed with related triazolium tetrafluoroborate salts. Variation of catalyst and reaction atmosphere provides mechanistic insights, and revealed dioxygen as the terminal oxidant and the iodine/iodide couple as key redox component in the catalytic dehydrogenation pathway. While molecular iodine is competent as a catalyst in its own right, the triazolium cation triples the reaction rate and reaches turnover frequencies up to 30 h−1, presumably through beneficial interactions of the electron-poor azolium π system and I2, which facilitate the electron transfer from the substrate to iodine and concomitant formation of I−. This acceleration is specific for triazolium cations and represents a hybrid anion/cation catalytic process as a simple and straightforward route towards imine products, with economic advantages over previously reported metal-based catalytic systems.
- This article is part of the themed collections: Hybrid Catalysis and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...