BF3·OEt2-mediated cyclization of β,γ-unsaturated oximes and hydrazones with N-(arylthio/arylseleno)succinimides: an efficient approach to synthesize isoxazoles or dihydropyrazoles†
Abstract
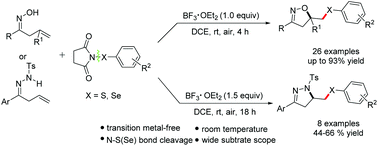
A highly efficient BF3·OEt2-mediated cyclization of β,γ-unsaturated oximes and tosylhydrazones with N-(arylthio/arylseleno)succinimides has been established for the construction of N-heterocycles in a one-step manner. This metal-free cyclization provides direct access to isoxazoles and dihydropyrazoles in good to excellent yields at room temperature. The mechanistic experiments support the formation of a cationic species PhS+ which plays a critical role in this cyclization process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...