An entry to non-racemic β-tertiary-β-amino alcohols, building blocks for the synthesis of aziridine, piperazine, and morpholine scaffolds†
Abstract
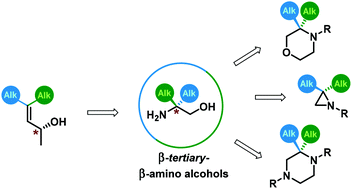
A method for the preparation of enantiopure β-tert-amino alcohols bearing a tetrasubstituted C-stereocenter, as well as their conversion into selected medicinally privileged heterocyclic systems (morpholines, aziridines, piperazines) is reported. These compounds were obtained through enantiospecific sigmatropic rearrangement of allyl carbamates as a key step. The latter were prepared from the corresponding β,β′-dialkyl-substituted non-racemic allyl alcohols. In addition, an asymmetric synthesis of such highly substituted allylic alcohols via either enantioselective 1,2-reduction of enones, enzymatic kinetic resolution, or a functionalization of chiral propargyl alcohols, with discussion of scope and limitations of each method is reported.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...