Access to a stabilized i-motif DNA structure through four successive ligation reactions on a cyclopeptide scaffold†
Abstract
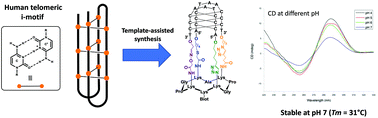
i-Motifs are largely underexplored tetraplex nucleic acid structures which have been suggested to perform essential biological functions and might constitute future therapeutic targets. i-Motifs generally require acidic conditions to fold in vitro, a particularity which significantly complicates the use of native i-motif forming sequences for interaction studies with potential ligands and biological components (e.g. proteins). In this context, we report herein on the assembly of a peptide–DNA conjugate capable of folding at room temperature into a stable i-motif structure at neutral pH. To achieve the controlled assembly of the i-motif forming conjugate, we developed a new synthetic pathway of four successive orthogonal ligation reactions between bifunctional C-rich DNA strands and a tetrafunctional cyclopeptide scaffold.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...