Substrate tolerance of the biosynthetic enzymes of glycosylated lanthipeptide NAI-112†
Abstract
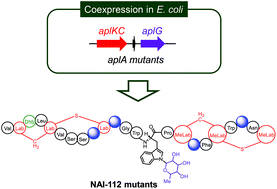
NAI-112 is a glycosylated class III lanthipeptide produced by an Actinoplanes sp. strain with potent bioactivity against nociceptive pain. It contains two labionin/methyllabionin motifs and a rare deoxyhexose modification N-linked to a tryptophan residue. In this study, we investigated the substrate tolerance of the biosynthetic machinery of NAI-112 by using a heterologous co-expression system in Escherichia coli. The results demonstrate AplKC as the first class III lanthipeptide synthetase to catalyze the formation of two labionin/methyllabionin motifs independently. As a rare Trp(N) glycosyltransferase, AplG shows the requirement of two intact ring structures in peptides for substrate recognition. Structural modelling and mutagenesis studies helped identify three residues of catalytic importance in AplG.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...