Gold-catalyzed dual C–C bond cleavage of biphenylenes bearing a pendant alkyne at ambient temperature†
Abstract
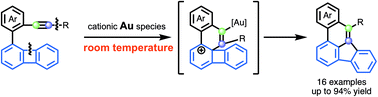
We report a catalytic skeletal rearrangement of biphenylenes with a pendant alkyne moiety at room temperature by a cationic gold catalyst, which involves the cleavage of two bonds: the C–C bond of biphenylene and the C(sp)–C(sp2 or sp3) bond. Experimental and theoretical studies revealed that the reaction mechanism included π-activation of the alkyne, ring expansion and 1,2-carbon shift.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...