Synthetic strategies in construction of organic macromolecular carrier–drug conjugates
Abstract
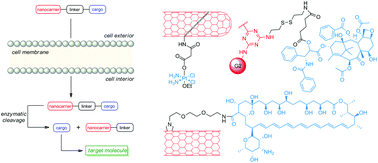
Many metabolic inhibitors, considered potential antimicrobial or anticancer drug candidates, exhibit very limited ability to cross the biological membranes of target cells. The restricted cellular penetration of those molecules is often due to their highhydrophilicity. One of the possible solutions to this problem is a conjugation of an inhibitor with a molecular organic nanocarrier. The conjugate thus formed should be able to penetrate the membrane(s) by direct translocation, endocytosis or active transport mechanisms and once internalized, the active component could reach its intracellular target, either after release from the conjugate or in an intact form. Several such nanocarriers have been proposed so far, including macromolecular systems, carbon nanotubes and dendrimers. Herein, we present a comprehensive review of the current status of rational design and synthesis of macromolecular organic nanocarrier-drug conjugates, with special attention focused on the mode of coupling of a nanocarrier moiety with a “cargo” molecule through linking fragments of non-cleavable or cleavable type.


 Please wait while we load your content...
Please wait while we load your content...