Recent advances in PIII-assisted deoxygenative reactions under photochemical or electrochemical conditions
Abstract
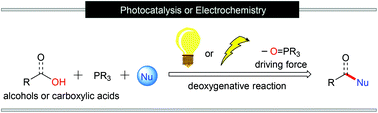
The nucleophilic substitution reactions of hydroxyl groups are one of the most fundamental and widely spread transformations in organic chemistry. Among them, PIII-mediated deoxygenative nucleophilic substitution reactions, such as the Mitsunobu reaction, are frequently used strategies and often require stoichiometric oxidants to activate PIII reagents to induce the desired reactions. It has been illustrated that PIII reagents can be oxidized into the corresponding radical cations through single-electron oxidation by photocatalysis or electro-oxidation. These phosphine radical cations can react with alcohols or carboxylic acids to form the corresponding alkoxyphosphonium or acyloxyphosphonium intermediates, which are very reactive and easily get decomposed. The release of tri-substituted phosphine oxides as a driving force triggers the following nucleophilic substitution. This strategy does not require the use of stoichiometric oxidants and it eludes safety and stability problems. In this review, we summarise the recent advances and discoveries in PIII-assisted direct deoxygenative reactions under photochemical or electrochemical conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...