A PPh3-catalyzed sequential annulation reaction to construct cyclopentane-fused dihydropyrazolone-pyrrolidinediones†
Abstract
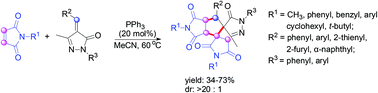
A PPh3-catalyzed sequential cycloaddition of maleimides with unsaturated pyrazolones has been developed. This protocol provides a simple and practical strategy for the construction of dispirocyclopentyl-[dihydropyrazolone-pyrrolidinedione] pyrrolidinedione skeletons containing five contiguous stereogenic centers, including two spiro-quaternary centers, with moderate yields (34–73%) and excellent diastereoselectivities (>20 : 1).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...