Synthesis of hydrophilic caged DAG-lactones for chemical biology applications†
Abstract
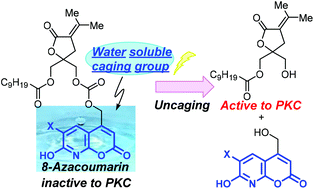
The 6-bromo-7-hydroxy-coumarin-4-ylmethyl (Bhc) group has been used widely in cage chemistry because of its high molar absorptivity and photolytic efficiency. One of the drawbacks of coumarins however is their low aqueous solubility. Aqueous solubility is important in the behavior of caged compounds because hydrophobic caged compounds might be aggregated in physiological conditions and consequently the photocleavage would be impaired. The 8-azacoumarin-4-ylmethyl derivatives with bromine (8-aza-Bhc) or iodine (8-aza-Ihc), which were previously developed in this laboratory, have aqueous solubilities that are higher than those of related coumarins. Here, to improve the hydrophilicity and management of caged diacylglycerol lactones (DAG-lactones), 8-aza-Bhc and 8-aza-Ihc were introduced into the DAG-lactone structure. The synthesized caged compounds showed high hydrophilicity compared with the parent Bhc-caged DAG-lactone, and the 8-aza-Ihc-caged DAG-lactone (2) showed excellent photolytic efficiency, which allows rapid release of the DAG-lactone (1) by brief photoirradiation. The 8-aza-7-hydroxy-6-iodo-coumarin-4-ylmethyl group might be useful for caging of bioactive compounds, especially hydrophobic compounds.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...