O-Cyclopropyl hydroxylamines: gram-scale synthesis and utility as precursors for N-heterocycles†
Abstract
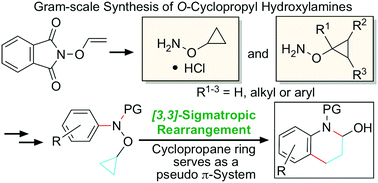
O-Cyclopropyl hydroxylamines, now accessible via a novel and scalable synthetic route, have been demonstrated to be bench-stable and practical precursors for the synthesis of N-heterocycles via a di-heteroatom [3,3]-sigmatropic rearrangement. In order to study the reactivity of these compounds in depth, a robust synthesis of both ring-substituted and ring-unsubstituted O-cyclopropyl hydroxylamines has been developed. Metal-free conditions for the facile N-arylation of these precursors were also identified. It was found that the N-arylated O-cyclopropyl hydroxamates can efficiently undergo a one-pot [3,3]-sigmatropic rearrangement/cyclization/rearomatization cascade under base-mediated conditions to furnish a structurally diverse set of substituted tetrahydroquinolines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...