A dearomative ipso-iodocyclization/desymmetrization sequence leading to optically active tricyclic piperazine scaffolds†
Abstract
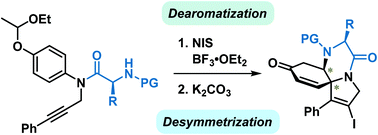
Dearomative ipso-iodocyclization of 4-(1-ethoxyethoxy)-N-propargylanilines leading to 1-azaspiro[4.5]deca-3,6,9-trien-8-ones has been developed. This reaction is characterized by the yield of fewer toxic byproducts and is conducted by a more user-friendly protocol compared to other reported methods. The synthetic application of these spiro products was demonstrated by transformation of the iodo component into other functionalities. The desymmetrization of the resulting cyclohexadienones was also achieved by the diastereoselective aza-Michael addition of internal amino acid moieties to yield tricyclic piperazine scaffolds with two newly formed contiguous chiral centers.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...