Room temperature nickel-catalyzed cross-coupling of aryl-boronic acids with thiophenols: synthesis of diarylsulfides†
Abstract
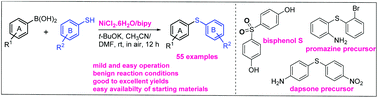
A NiCl2/2,2′-bipyridine-catalyzed cross-coupling of thiophenols with arylboronic acids has been developed for the synthesis of symmetric and unsymmetric diarylsulfides at room temperature and in air. This methodology is reliable and offers a mild and easy to operate process for the synthesis of arylthioethers, which are essential compounds with applications in the pharmaceutical and agricultural industries. This method avoids the use of expensive transition metals, such as Pd, Ir or Rh, sophisticated ligands and elevated temperatures. It also has a wide substrate scope (55 examples) and provides products in good to excellent yields (72–93%).
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...