Nicotinamide riboside–amino acid conjugates that are stable to purine nucleoside phosphorylase†
Abstract
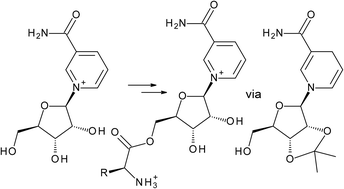
The nutraceutical Nicotinamide Riboside (NR), an efficacious biosynthetic precursor to NAD, is readily metabolized by the purine nucleoside phosphorylase (PNP). Access to the PNP-stable versions of NR is difficult because the glycosidic bond of NR is easily cleaved. Unlike NR, NRH, the reduced form of NR, offers sufficient chemical stability to allow the successful functionalisation of the ribosyl-moiety. Here, we report on a series of NRH and NR derived amino acid conjugates, generated in good to excellent yields and show that O5′-esterification prevents the PNP-catalyzed phosphorolysis of these NR prodrugs.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...