Access to C4-arylated benzoxazoles from 2-amidophenol through C–H activation†
Abstract
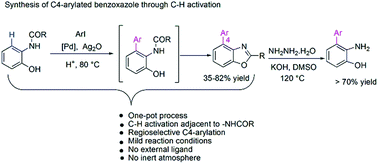
A Pd-catalyzed aerobic approach to access C4-aryl benzoxazoles by tandem C–H ortho-arylation and acid-mediated annulation of 2-amidophenol has been presented. The directing potential of the –NHCOR group over the –OH group was exploited for selective arylation adjacent to the amide group. Deuterium labeling experiments suggest that palladation predominantly occurs adjacent to the –NHCOR group and is the key step during benzoxazole formation. One-pot hydrolysis of the resulting C4-arylated benzoxazole was also accomplished to access structurally challenging 3-aryl aminophenols for further applications.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...