Rhodium(iii)-catalyzed ortho-C–H amidation of 2-arylindazoles with a dioxazolone as an amidating reagent†
Abstract
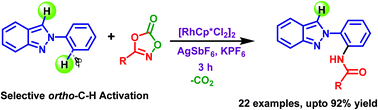
A simple and efficient method for the directed amidation of a wide range of 2-arylindazoles has been established for the first time through a rhodium-catalyzed C–H activation reaction with alkyl, aryl and heteroaryl dioxazolones. A series of N-(2-(2H-indazol-2-yl)phenyl)acetamide derivatives were synthesized in excellent yields. A mechanistic study was conducted to describe C–H bond cleavage which is likely to be involved in the rate determining step.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...