Hypervalent iodine promoted ortho diversification: 2-aryl benzimidazole, quinazoline and imidazopyridine as directing templates†
Abstract
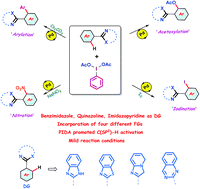
The mild and efficient palladium-catalyzed ortho C(sp2)–H diversification of (NH)-free 2-substituted benzimidazole, quinazoline, and imidazopyridine is reported using hypervalent iodine as the key reagent. Acetoxy, aryl, iodide and nitro functional groups were introduced on the same substrate by simply shifting the reaction conditions in the presence of inorganic additives (Cs2CO3, I2, NaNO2) and the hypervalent iodine reagent (diacetoxyiodo)benzene (PIDA) under aerobic conditions. The combination of NaNO2 with PIDA was successfully employed in Pd-catalyzed C–H bond nitration to achieve a library of nitrated 1,3 N-heterocycles. This versatile ortho C(sp2)–H activation strategy features operational simplicity, short reaction times, and ample substrate possibilities, it requires no ligands or silver salts as additives, and it shows good tolerance of oxidation prone functional groups.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...