The synthesis of unnatural α-alkyl- and α-aryl-substituted serine derivatives†
Abstract
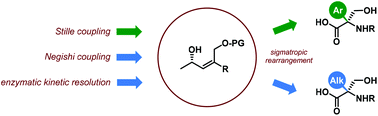
The synthesis of α-aryl- and α-alkyl-substituted serine derivatives via [3,3]-sigmatropic rearrangement of allyl carbamates as a key step is reported. Allyl carbamates were obtained from the corresponding allyl alcohols. The former were prepared through three approaches. Aryl-substituted ones were synthesized via the Stille coupling reaction of aryl iodides with enantiomerically enriched vinyl stannanes. Conversely, alkyl-substituted allyl alcohols were prepared by an analogous strategy involving the Negishi coupling reaction of enantiomerically enriched vinyl iodides or by enzymatic kineric resolution of the corresponding racemic alcohols.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...