Te–S covalent bond induces 1T&2H MoS2 with improved potassium-ion storage performance†
Abstract
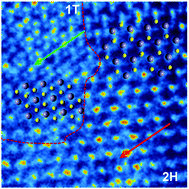
The modulation of the characteristics of an MoS2 anode via substitutional doping, particularly N, P and Se, is vital for promoting the potassium-ion storage performances. However, these traditional chalcogen doping can only take the place of a sulfur element and not essentially change the inherent electrical nature of MoS2. Herein, novel Te-MoS2 materials have been synthesized via a simple hydrothermal process under Te doping. A half-metallic Te occupies the position of an Mo atom to form Te–S bonds, which is different from the same group Se element. After theoretical modeling and electrochemical measurements, it was observed that the formation of Te–S bonds can increase the electrical conductivity (about 530 times increment) and mitigate the mechanical stress to ensure the whole structural stability during the repeated insertion/extraction of K-ions. Moreover, the insertion of Te into the lattice of MoS2 generated the fractional phase transformation from 2H to the 1T phase of MoS2 and 1T&2H in-plane hetero-junction. Benefiting from these advantages, the 1T&2H Te-MoS2 anode delivered high capacities of 718 and 342 mA h g−1 at 50 and 5000 mA g−1, respectively, and an ultra-stable cycling performance (88.1% capacity retention after 1000 cycles at 2 A g−1). Moreover, the potassium-ion full cell assembled with K2Fe[Fe(CN)6] as the cathode demonstrates its practical application.


 Please wait while we load your content...
Please wait while we load your content...