The hydrogen-bond configuration modulates the energy transfer efficiency in helical protein nanotubes†
Abstract
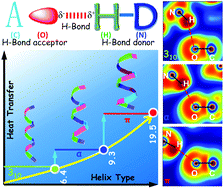
Energy transport in proteins is critical to a variety of physical, chemical, and biological processes in living organisms. While strenuous efforts have been made to study vibrational energy transport in proteins, thermal transport processes across the most fundamental building blocks of proteins, i.e. helices, are not well understood. This work studies energy transport in a group of “isomer” helices. The π-helix is shown to have the highest thermal conductivity, 110% higher than that of the α-helix and 207% higher than that of the 310-helix. The H-bond connectivity is found to govern thermal transport mechanisms including the phonon spectral energy density, dispersion, mode-specific transport, group velocity, and relaxation time. The energy transport is strongly correlated with the H-bond strength which is also modulated by the H-bond connectivity. These fundamental insights provide a novel perspective for understanding energy transfer in proteins and guiding a rational molecule-level design of novel materials with configurable H-bonds.


 Please wait while we load your content...
Please wait while we load your content...
