Controlling palladium morphology in electrodeposition from nanoparticles to dendrites via the use of mixed solvents†
Abstract
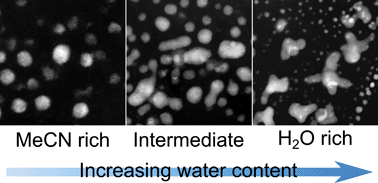
By changing the mole fraction of water (χwater) in the solvent acetonitrile (MeCN), we report a simple procedure to control nanostructure morphology during electrodeposition. We focus on the electrodeposition of palladium (Pd) on electron beam transparent boron-doped diamond (BDD) electrodes. Three solutions are employed, MeCN rich (90% v/v MeCN, χwater = 0.246), equal volumes (50% v/v MeCN, χwater = 0.743) and water rich (10% v/v MeCN, χwater = 0.963), with electrodeposition carried out under a constant, and high overpotential (−1.0 V), for fixed time periods (50, 150 and 300 s). Scanning transmission electron microscopy (STEM) reveals that in MeCN rich solution, Pd atoms, amorphous atom clusters and (majority) nanoparticles (NPs) result. As water content is increased, NPs are again evident but also elongated and defected nanostructures which grow in prominence with time. In the water rich environment, NPs and branched, concave and star-like Pd nanostructures are now seen, which with time translate to aggregated porous structures and ultimately dendrites. We attribute these observations to the role MeCN adsorption on Pd surfaces plays in retarding metal nucleation and growth.


 Please wait while we load your content...
Please wait while we load your content...