Revealing the sub-50 ms electrochemical conversion of silver halide nanocolloids by stochastic electrochemistry and optical microscopy†
Abstract
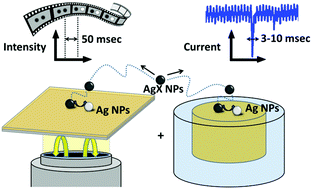
Silver based ionic crystal nanoparticles (NPs) are interesting nanomaterials for energy storage and conversion, e.g. their colloidal solutions could be used as a reversible redox nanofluid in semi-solid redox flow cells. In this context, the reductive transformation of Brownian silver halide, AgX, NPs into silver NPs is probed by single NP electrochemistry, complemented by operando high resolution monitoring. However, their light sensitivity and poor conductivity make the operando monitoring of their chemical activity challenging. The electrochemical collisions of single AgX NPs onto a negatively biased electrode evidence a full conversion through multiple reduction steps within 3–10 ms. This is further corroborated by simulation of the conversion process and operando through a high resolution optical microscopy technique (Backside Absorbing Layer Microscopy, BALM). Both techniques are interesting strategies to infer at the single NP level the intrinsic charge capacity and charging rate of redox active Brownian nanomaterials, demonstrating the interest of the fast and reversible AgX/Ag system as a redox nanofluid.
- This article is part of the themed collection: 2020 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...