In situ unraveling of the effect of the dynamic chemical state on selective CO2 reduction upon zinc electrocatalysts†
Abstract
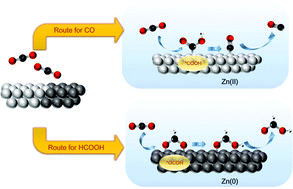
Unraveling the reaction mechanism behind the CO2 reduction reaction (CO2RR) is a crucial step for advancing the development of efficient and selective electrocatalysts to yield valuable chemicals. To understand the mechanism of zinc electrocatalysts toward the CO2RR, a series of thermally oxidized zinc foils is prepared to achieve a direct correlation between the chemical state of the electrocatalyst and product selectivity. The evidence provided by in situ Raman spectroscopy, X-ray absorption spectroscopy (XAS) and X-ray diffraction significantly demonstrates that the Zn(II) and Zn(0) species on the surface are responsible for the production of carbon monoxide (CO) and formate, respectively. Specifically, the destruction of a dense oxide layer on the surface of zinc foil through a thermal oxidation process results in a 4-fold improvement of faradaic efficiency (FE) of formate toward the CO2RR. The results from in situ measurements reveal that the chemical state of zinc electrocatalysts could dominate the product profile for the CO2RR, which provides a promising approach for tuning the product selectivity of zinc electrocatalysts.
- This article is part of the themed collection: Advanced Nanomaterials for Energy Conversion and Storage


 Please wait while we load your content...
Please wait while we load your content...