A combined experimental and theoretical approach revealing a direct mechanism for bifunctional water splitting on doped copper phosphide†
Abstract
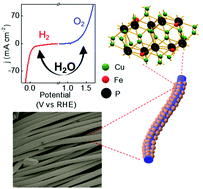
A cost-effective electrocatalyst should have a high dispersion of active atoms and a controllable surface structure to optimize activity. Additionally, bifunctional characteristics give an added benefit for the overall water splitting. Herein, we report the synthesis and fabrication of Fe-doped Cu/Cu3P supported on a flexible carbon cloth (CC) with a hydrophilic surface for efficient bifunctional water electrolysis under alkaline conditions. Surface doping of Fe in the hexagonal Cu3P does not alter the lattice parameters, but it promotes the surface metallicity by stimulating Cuδ+ and Cu0 sites in Cu3P, resulting in an augmented electroactive surface area. Cu2.75Fe0.25P composition exhibits unprecedented OER activity with a low overpotential of 470 mV at 100 mA cm−2. Under a two electrode electrolyzer system the oxygen and hydrogen gas was evolved with an unprecedented rate at their respective electrode made of same catalyst. Density functional theory further elucidates the role of the Fe center toward electronic state modulation, which eventually alters the entire adsorption behavior of the reaction intermediates and reduces the overpotential on Fe-doped system over pristine Cu3P.


 Please wait while we load your content...
Please wait while we load your content...