Synthesis of monodisperse water-stable surface Pb-rich CsPbCl3 nanocrystals for efficient photocatalytic CO2 reduction†
Abstract
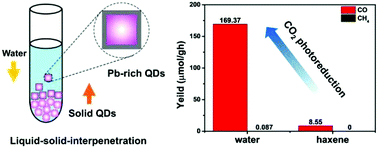
Surface Pb-rich lead halide (CsPbCl3) perovskite nanocrystals (NCs) with high stability and monodispersity in water have been synthesized using a general and convenient liquid–solid interpenetration (LSI) method. In this process, water molecules permeate into the solid CsPbCl3 NC layers and slowly dissolve the Cs+ and Cl− ions on the surface of CsPbCl3 NCs. The Cs+ and Cl− ions in water inhibit the decomposition rate of CsPbCl3 NCs, inducing surface Pb-rich layers. The surface Pb-rich structure increases the photoluminescence (PL) lifetimes and improves the photocatalytic performances of lead halide perovskite NCs. Under simulated solar irradiation, the largest rate of CO2 photoreduction from surface Pb-rich Ni-doped CsPbCl3 NCs reaches up to 169.37 μmol g−1 h−1. This study provides an effective general strategy to design stable lead halide perovskite quantum dots (QDs) for their wide applications.


 Please wait while we load your content...
Please wait while we load your content...