Intercalation of laminar Cu–Al LDHs with molecular TCPP(M) (M = Zn, Co, Ni, and Fe) towards high-performance CO2 hydrogenation catalysts†
Abstract
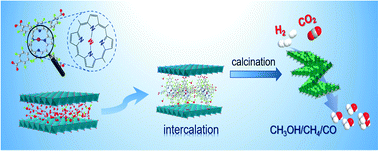
A confined space is broadly applied to enhance the dispersion and limit the aggregation of catalytically active sites, especially at high temperatures. In this work, we provided an efficient approach to immobilize transition metal ions (e.g., Zn2+, Co2+, Ni2+, and Fe2+) into the confined space of laminar Cu–Al layered double hydroxides (LDHs) using a range of molecular metalloporphyrins (viz., TCPP(M)) as shuttles. The deprotonated TCPP(M) not only provides nitrogen-based coordination sites to anchor a series of transition metal ions, but also intercalates and diffuses facilely into the interlayer gallery of LDHs by ion exchange. The obtained TCPP(M)@Cu–Al LDHs were then used as solid precursors for the fabrication of a series of heterogeneous catalysts for CO2 hydrogenation via high-temperature calcination. Two restriction forces contributed to the enhanced dispersion of the active species over the catalyst surface structures. Remarkably, the transition metals positioned within the confined space of LDHs significantly affected the catalytic performance of CO2 hydrogenation. Mainly CO, methanol, and methane were found as the C1 products, and their selectivities are highly dependent on the reaction intermediates, as suggested by the in situ DRIFTS study. Moreover, the designed catalysts fabricated via molecular TCPP(M) intercalation exhibited much better performance than the conventional catalysts derived from surface-supported CA-LDHs, due to their better metal dispersion and smaller particle size.


 Please wait while we load your content...
Please wait while we load your content...