Boosted electrochemical ammonia synthesis by high-percentage metallic transition metal dichalcogenide quantum dots†
Abstract
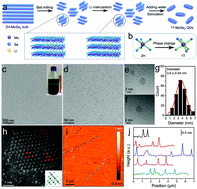
The electrochemical method can directly convert N2 into the high-value-added NH3 under ambient conditions and is considered to be a green and sustainable alternative to the traditional Haber–Bosch process. However, the electrochemical nitrogen reduction reaction (NRR) suffers from a low ammonia yield rate over the reported electrocatalysts. Herein, we have developed a general strategy to boost the NRR performance, enabled by the metallic 1T phase dominated transition metal dichalcogenide quantum dots (TMD QDs). Impressively, the obtained MoSe2 QDs achieved a superior ammonia yield rate of 340 μg mg−1 cat. h−1 with excellent ammonia generation sustainability. Experimental and theoretical studies revealed that the excellent catalytic activity of MoSe2 QDs mainly originates from the ultra-small quantized size (high surface area and high-density active edge/defect sites) and high-percentage metallic 1T phase (the N2 adsorption on the 1T phase is spontaneous, and the energy barrier of the potential determining step on the 1T phase is very low). Most importantly, our concept is universal for TMD materials (i.e., MoS2, WSe2, WS2 and NbSe2) that also exhibit a much-enhanced ammonia yield rate as compared to other electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...