Iron-mediated interaction of alpha synuclein with lipid raft model membranes†
Abstract
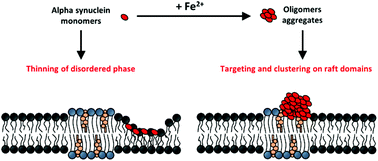
The aberrant misfolding and aggregation of alpha synuclein (αS) into toxic oligomeric species is one of the key features associated with the pathogenesis of Parkinson's disease (PD). It involves different biochemical and biophysical factors as plasma membrane binding and interaction with heavy metal ions. In the present work, atomic force microscopy (AFM) is combined with Fourier Transform Infrared Spectroscopy (FTIR) measurements to investigate the interaction of wild-type (WT) and A53T mutated alpha synuclein with artificial lipid bilayers mimicking lipid raft (LR) domains, before and after ferrous cations (Fe2+) treatment. In the absence of iron, protein monomers produce a thinning of the membrane, targeting the non-raft phase of the bilayer preferentially. On the contrary, iron actively promotes the formation of globular protein aggregates, resembling oligomers, targeted to LR domains. In both aggregation states, monomer and oligomer, the mutated A53T protein exhibits a greater and faster membrane-interaction. These results underlie a new mechanism of membrane-protein interaction in PD. The targeting of Fe2+-promoted αS oligomers to LRs might be functional for the disease and be helpful for the development of new therapeutic strategies.


 Please wait while we load your content...
Please wait while we load your content...