A model beyond protein corona: thermodynamics and binding stoichiometries of the interactions between ultrasmall gold nanoclusters and proteins†
Abstract
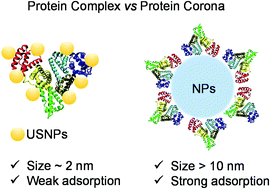
Nanoparticles (NPs) will inevitably interact with proteins and form protein coronas once they are exposed to biological fluids. This conventional model for nano–bio interactions has been used for over twenty years. Growing numbers of new nanomaterials are emerging every year. Among them, noble metal nanoclusters (NMNCs) are new types of fluorescent nanomaterials with considerable advantages in biomedical applications. Compared with NPs (typically >10 nm) like Au NPs, carbon nanotubes, etc., NMNCs have ultrasmall sizes (∼2 nm), so when NMNCs are exposed to biological milieu, will they form protein coronas like NPs? Due to a lack of characterization techniques for ultrasmall nanoparticles (USNPs), to date, studies on the binding stoichiometries of USNPs to proteins have been heavily hampered. To address this challenge, we combined the characteristics of various methods and selected human serum albumin (HSA) and transferrin (Trf) as model proteins to study their interactions with dihydrolipoic acid (DHLA) protected gold nanoclusters (DHLA-AuNCs). Steady-state fluorescence, transient fluorescence spectroscopy and isothermal titration calorimetry (ITC) were used to study the thermodynamic parameters (K, ΔH, ΔS, ΔG) and interaction mechanisms. The results showed that the intrinsic fluorescence of both proteins was quenched by DHLA-AuNCs, and the quenching process of HSA was an endothermic dynamic process. In contrast, the quenching process of Trf was an exothermic static process. The combination of ITC, agarose gel electrophoresis (AGE) and zeta potential showed that one HSA could bind 8 ± 1 DHLA-AuNCs and one Trf could bind 7 ± 2 DHLA-AuNCs, which was quite different from the conventional model of protein coronas. Based on these findings, the “protein complex” was termed for proteins upon binding with USNPs. Dynamic light scattering (DLS), transmission electron microscopy (TEM), and atomic force microscopy (AFM) showed that DHLA-AuNCs could induce the agglomeration of proteins. Circular dichroism (CD) and synchronous fluorescence spectroscopy showed that DHLA-AuNCs had a very minor effect on the secondary structures of HSA and Trf, which demonstrated the good biocompatibility of DHLA-AuNCs at the molecular scale. This work has shed light on a new interaction model beyond the protein corona, indicating a possible biological identity of USNPs.


 Please wait while we load your content...
Please wait while we load your content...