Meroterpenoid natural products from Streptomyces bacteria – the evolution of chemoenzymatic syntheses
Abstract
Covering: Up to January 2020
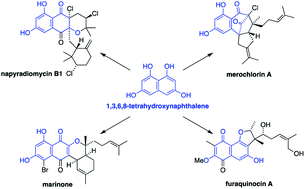
Meroterpenoids derived from the polyketide 1,3,6,8-tetrahydroxynaphthalene (THN) are complex natural products produced exclusively by Streptomyces bacteria. These antibacterial compounds include the napyradiomycins, merochlorins, marinones, and furaquinocins and have inspired many attempts at their chemical synthesis. In this review, we highlight the role played by biosynthetic studies in the stimulation of biomimetic and, ultimately, chemoenzymatic total syntheses of these natural products. In particular, the application of genome mining techniques to marine Streptomyces bacteria led to the discovery of unique prenyltransferase and vanadium-dependent haloperoxidase enzymes that can be used as highly selective biocatalysts in fully enzymatic total syntheses, thus overcoming the limitations of purely chemical reagents.
- This article is part of the themed collection: Enzymes in natural product total synthesis


 Please wait while we load your content...
Please wait while we load your content...